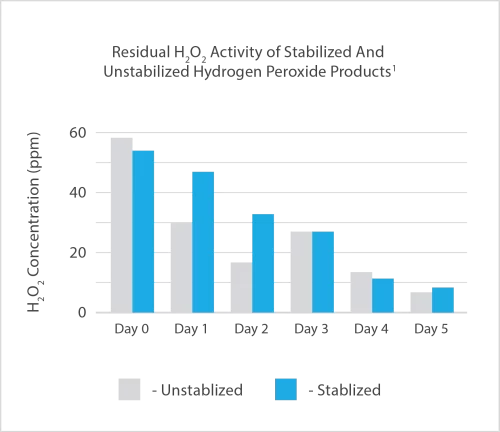
Oxidising biocides such as ozone, hydrogen peroxide and peracetic acid are known for their instability and difficulty in safely handling and applying.
Chlorine Dioxide belongs to the same family of biocides as the oxidising biocides, sharing more in common with them than its “chlorine” namesake.
Chlorine Dioxide has several advantages over other oxidising biocides, making it more suitable for many water treatment applications.
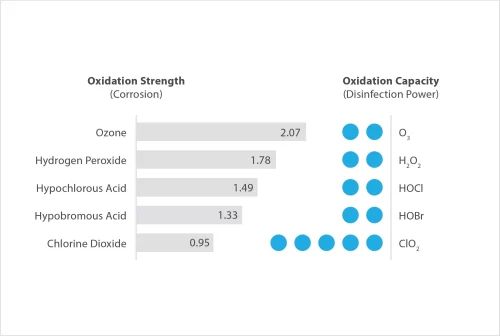
When compared with other oxidising biocides, Chlorine Dioxide has a significantly lower oxidation strength – this means that it reacts with fewer compounds, such as organic compounds and ammonia, yet is strong enough to attack the disulphide bonds found in the membranes of bacteria and other biological material.
This “selective oxidation” process allows the Chlorine Dioxide biocide to be targeted where it is needed most, disinfecting areas quickly and at lower dose rates, leading to greater cost efficiencies.
As an example, where Hydrogen Peroxide-based products have been promoted for use in water treatment, this is often at dose rates 10-30 times greater than ClO2, leading to difficulties in handling peroxide-based products >15% that are now listed as “Explosives Precursors” under EU Regulations.