While chlorine dioxide has “chlorine” in its name, its chemistry is radically different from that of chlorine.
As we all learned in high school chemistry, we can mix two compounds and create a third that bears little resemblance to its parents.
For instance, by mixing two parts of hydrogen gas with one of oxygen – liquid water is the formed. We should not be misled by the fact that chlorine and chlorine dioxide share a word in common. The chemistries of the two compounds are completely different.
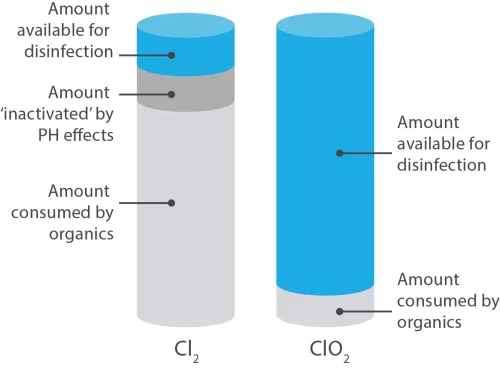
Chlorine and chlorine dioxide are both oxidising agents (electron receivers). However, chlorine has the capacity to take in two electrons, whereas chlorine dioxide can absorb five. This means that, mole for mole, ClO2 is 2.6 times more effective than chlorine.
If equal, if not greater importance is the fact that chlorine dioxide will not react with many organic compounds, and as a result ClO2 does not produce environmentally dangerous chlorinated organics. For example; aromatic compounds have carbon atoms arranged in rings and they may have other atoms, such as chlorine, attached to these rings, to form a chlorinated aromatic – a highly toxic compound that persists in the environment long after it is produced.
Chlorine dioxide’s behaviour as an oxidising agent is quite dissimilar. Like ozone, the predominant oxidation reaction mechanism for chlorine dioxide proceeds through a process known as free radical electrophilic (i.e. electron-attracting) abstraction rather than by oxidative substitution or addition (as in chlorinating agents such as chlorine or hypochlorite). This means that chlorinated organic compounds such as THMs and HAAs are not produced as a result of disinfection using chlorine dioxide.